Gas Detector is a device that detects the presence of gases in an area. This type of equipment is used to detect a gas leak or other emissions and can interface with a control system so a process can be automatically shut down or giving the humans the opportunity to leave. The five most commonly recognized gas detection technologies, regardless of their detected gas type, Flammable, Combustible or Toxic, are Catalytic, Infrared (IR), Open Path, Electrochemical and Semiconductor Gas Detectors.
Catalytic Bead (Pellistor) Gas Detector: |
|
|
|
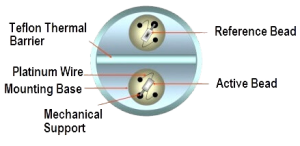
It is used primarily to detect combustible gases. Catalytic gas detectors are based upon the principle that when gas oxidizes it produces heat, and the sensor converts the temperature change via a standard Wheatstone Bridge type circuit to a sensor signal that is proportional to the gas concentration. The sensor components consist of a pair of heating coils (reference and active).The active element is embedded in a catalyst. The reaction takes place on the surface of the catalyst, with combustible gases reacting exothermically with oxygen in the air to raise its temperature. This results in a change of resistance. There is also a reference element providing an inert reference signal by remaining non-responsive to gas, thereby acting as a stable baseline signal to compensate for environmental changes which would otherwise affect the sensor s temperature.
|
Advantages+ Long life with a low replacement cost. + Less sensitive to temperature, humidity, condition and pressure changes + High accuracy + Fast response + Monitors a wide range of combustible gases and vapors in air |
Disadvantages– Subject to sensor poisoning – Requires air or oxygen – Shortened life with frequent or continuous exposure to high LELs. |
Infrared (IR) Gas Detector: |
|
|
|
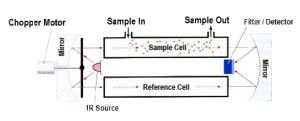
The Infrared (IR) detection method is based upon the absorption of infrared radiation at specific wavelengths as it passes through a volume of gas. Typically two infrared light sources and an infrared light detector measures the intensity of two different wavelengths, one at the absorption wavelength and one outside the absorption wavelength. If a gas intervenes between the source and the detector, the level of radiation falling on the detector is reduced. Gas concentration is determined by comparing the relative values between the two wavelengths. This is a dual beam infrared detector. Infrared gas detection is based upon the ability of some gases to absorb IR radiation. Many hydrocarbons absorb IR at approximately 3.4 micrometers and in this region H2O and CO2 are relatively transparent. As mentioned earlier, there are some hydrocarbons and other flammable gases that have poor or no response on a general purpose IR sensor. In addition to aromatics and acetylene, hydrogen, ammonia and carbon monoxide also cannot be detected using IR technology with general purpose sensors of 3.4 micron specifications.
|
Advantages+ High accuracy and selectivity + Large measurement range + Low maintenance + Highly resistant to chemical poisons + Does not require oxygen or air + No routine calibration required + Fail-to-safe |
Disadvantages– Not suitable for hydrogen detection |
Open Path (Line of Sight) Gas Detector: |
|
|
|
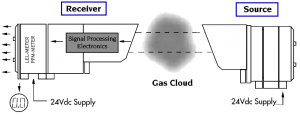
An alternative method of measuring gas concentration is based on absorption of infrared (IR) radiation at certain wavelengths as it passes through a volume of gas. Devices using this technology have a light source and a light detector and measure the light intensity at two specific wavelengths, one at an absorption (active) wavelength and one outside of the absorption (reference) wavelength. If a volume of gas passes between the source and detector, the amount of light in the active wavelength falling on the detector is reduced, while the amount of light in the reference wavelength remains unchanged. Much like the catalytic detectors, the gas concentration is determined from the relative difference between the two signals.
Open path IR detection offers immunity to poisons, high sensitivity gas leak detection, hazardous level gas detection, low maintenance, easy installation and fail to safe operation. However, it is not an all-encompassing answer to combustible gas detection. It offers an alternative solution to gas detection challenges and should be used in combination with point gas detection due to its limitations in targeting the specific location of gas leaks. |
Advantages+ High accuracy and selectivity + Large measurement range + Low maintenance + Highly resistant to chemical poisons + Does not require oxygen or air + No routine calibration required + Fail-to-safe |
Disadvantages– Not suitable for hydrogen detection – Is not capable of isolating the leak source (compare with point IR detection) – Requires unobstructed path between source and detector (compare with point IR detection) |
Electrochemical Gas Detector: |
|
|
|
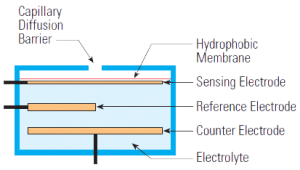
Electrochemical sensors operate by reacting with the gas of interest and producing an electrical signal proportional to the gas concentration.A typical electrochemical sensor consists of a sensing electrode (or working electrode), and a counter electrode separated by a thin layer of electrolyte. Gas that comes in contact with the sensor first passes through a small capillary type opening and then diffuses through a hydrophobic barrier, and eventually reaches the electrode surface. This approach is adopted to allow the proper amount of gas to react at the sensing electrode to produce a sufficient electrical signal while preventing the electrolyte from leaking out of the sensor. The gas that diffuses through the barrier reacts at the surface of the sensing electrode involving either an oxidation or reduction mechanism. These reactions are catalyzed by the electrode materials specifically developed for the gas of interest. With a resistor connected across the electrodes, a current proportional to the gas concentration flows between the anode and the cathode. The current can be measured to determine the gas concentration. Because a current is generated in the process, the electrochemical sensor is often described as an amperometric gas sensor or a micro fuel cell.
|
Advantages+ High sensitivity + Linear output + Easy to handle |
Disadvantages– Limited Shelf life – Subject to interference – Sensor lifetime shortened in very hot environment |
Metal Oxide Semiconductor (MOS) Gas Detector: |
|
|
|
A solid-state sensor consists of one or more metal oxides from the transition metals, such as tin oxide, aluminum oxide, etc. These metal oxides are prepared and processed into a paste which is used to form a bead-type sensor. Alternatively, thick or thin film-chip sensors are made when the metal oxides are vacuum deposited onto a silica chip, in a fashion similar to making semiconductors.A heating element is used to regulate the sensor temperature, since the finished sensors exhibit different gas response characteristics at different temperature ranges. This heating element can be a platinum or platinum alloy wire, a resistive metal oxide, or a thin layer of deposited platinum. The sensor is then processed at a specific high temperature which determines the specific characteristics of the finished sensor. In the presence of gas, the metal oxide causes the gas to dissociate into charged ions or complexes which results in the transfer of electrons. The builtin heater, which heats the metal oxide material to an operational temperature range that is optimal for the gas to be detected, is regulated and controlled by a specific circuit. A pair of biased electrodes are imbedded into the metal oxide to measure its conductivity change. The changes in the conductivity of the sensor resulting from the interaction with the gas molecules is measured as a signal. Typically, a solid-state sensor produces a very strong signal, especially at high gas concentrations.
|
Advantages+ High sensitivity + Wide operating temperature range + Long life |
Disadvantages– Non-specific (cross-sensitive to other compounds) – nonlinear output – Sensitive to changes in humidity – Subject to poisoning |
Comparison
Various technologies of Gas Detector pros and cons can be concluded as below:
| Detector type | Advantages | Disadvantages |
| Catalytic |
+ Simple, measure flammability of gases. + Low cost proven technology. |
– Can be poisoned by lead, chlorine and silicones that remains an unrevealed failure mode. – Requires oxygen or air to work. – High power. – Positioning critical. |
| Electrochemical |
+ Measures toxic gases in relatively low concentrations. + Wide range of gases can be detected. + Very low power. |
– Failure modes are unrevealed unless advanced monitoring techniques used. – Requires oxygen to work. – Positioning critical. |
| Point Infrared |
+ Uses a physical rather than chemical techniques. + Less sensitive to calibration errors. + No unseen failure modes. + Can be used in inert atmospheres. |
– Flammable gas detection only in %LEL range. – Measures concentration of flammable gases which have then to be related to the flammability of the gas. – Positioning critical. – High/medium power. |
| Open Path Infrared |
+ Area coverage-best chance to see a leak. + No unseen failure modes. + Latest technology. + Can detect low concentrations. + Positioning not as critical. + New toxic version as well as flammable. |
– Higher initial purchases cost. – Not suitable for use in smaller areas. – Detection path can be obscured. |
| Semiconductor |
+ Mechanically robust. + Works well in constant high humidity conditions. |
– Susceptible to contaminations and changes in environmental conditions. – Not linear response effects complexity. |
Special Applications
Portable Gas Detector: |
|
  |
Portable gas detectors are designed for team protection or area surveillance for short-term work where fixed gas systems are not suitable. These units refer to gas detectors which are worn or carried by an individual. Typically battery operated, portable monitors are used for toxic or combustible gas detection, as well as for oxygen deficiency monitoring in confined spaces. Ideal for confined spaces, spot leak testing and mobile use, portable gas detectors are marked by flexibility and quality. A variety of single or multi-gas detectors are offered in compact, lightweight designs, fully configurable and serviceable instruments. Applications include underground utilities vaults, boiler rooms, post-fire sites, sewers, industrial plants, industrial hygiene, first responder crews and remote fleets. |
Applicable Notes
- To positioning the Gas Detectors, related standards such as NFPA 72 or EN54 shall be considered.
- There are some terms which is used generally about gas detectors:
- LEL: Lower Explosive Limit. The minimum concentration of a combustible gas or vapor in air which will ignite if a source of ignition is present
- UEL: Upper Explosive Limit. Most, but not all, combustible gases have an upper explosive limit which is the maximum concentration in air which will support combustion.
- PPM: Parts Per Million (toxic & VOC)
- %VOL: Percent by volume (oxygen)
- VOC: Volatile Organic Compounds (PID)
- PID: Photo Ionization Detection (VOC)
- TWA: Time Weighted Average (toxic gases)
- STEL: Short Term Exposure Limit
- T90: Time sensor needs to reach 90% full response
- A bump test is a brief exposure of the sensor to gas and verifies if the sensor is responding and the alarm is functioning.
- Lower flammability limit (LFL) usually expressed in volume percent, is the lower end of the concentration range over which a flammable mixture of gas or vapor in air can be ignited at a given temperature and pressure. The flammability range is delineated by the upper and lower flammability limits. Outside this range of air/vapor mixtures, the mixture cannot be ignited (unless the temperature and pressure are increased).
- In environments with combustible gas hazards, it is important to know long before the gas concentration reaches the LEL. Typical safety standards require that a gas detection unit give warnings at 10 – 20% of the LEL. Do not confuse the alarm level with the volume of gas required to reach the LEL. For example: Methane has an LEL of 5% by volume in air. For a gas detector to give an alarm at 10% of the LEL, it must trigger when it detects 0.5% by volume. The detector for this application would most likely be calibrated for the range from 0% to 5% gas by volume, but display the reading as 0 – 100% LEL.
Our Services
SISICO is able to offer an extensive range of Gas Detectors to suit your specific environment from below mentioned companies: